Introduction :
`=>` Matter is made up of one or different type of elements.
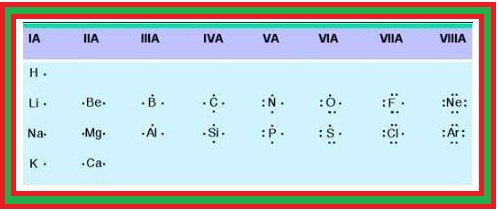
`=>` Under normal conditions no other element exists as an independent atom in nature, except noble gases.
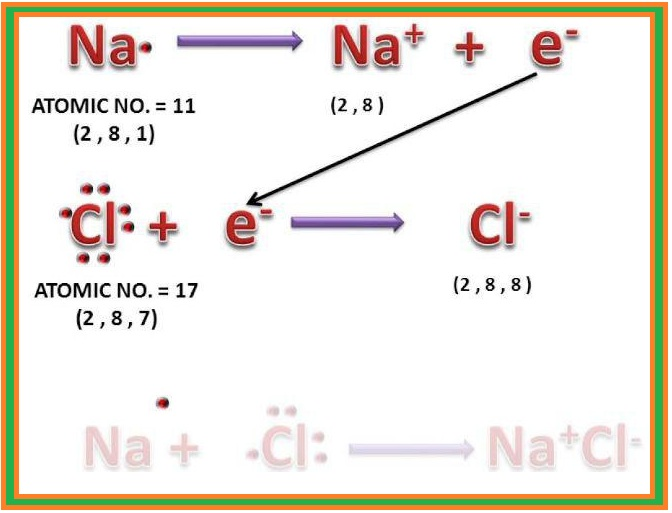
`=>` However, a group of atoms is found to exist together as one species having characteristic properties. Such a group of atoms is called a `color{red}("molecule")`.
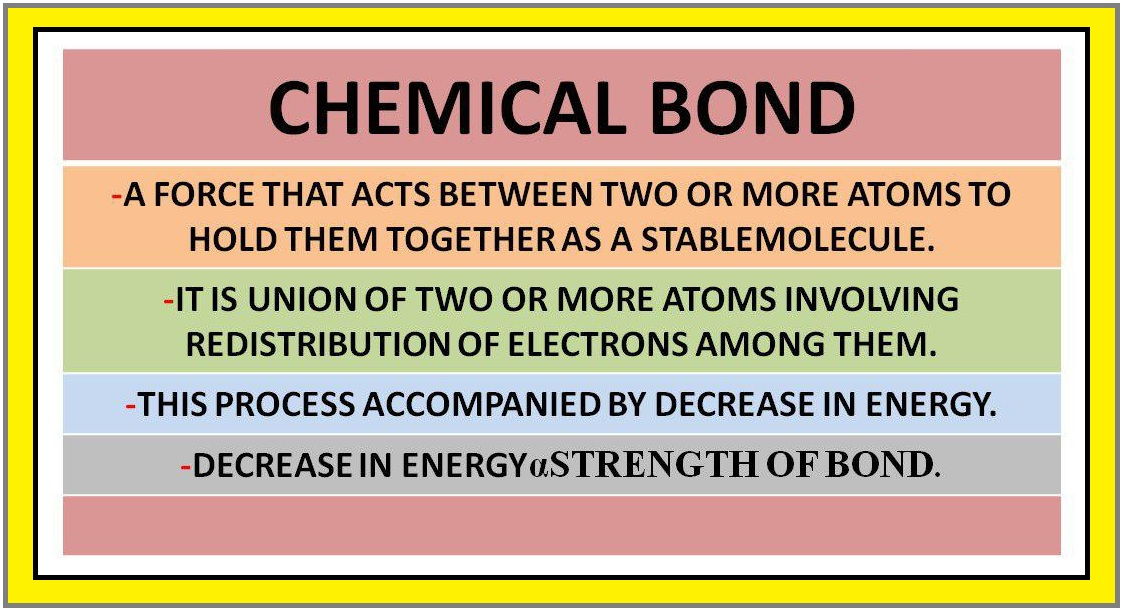
● Obviously there must be some force which holds these constituent atoms together in the molecules.
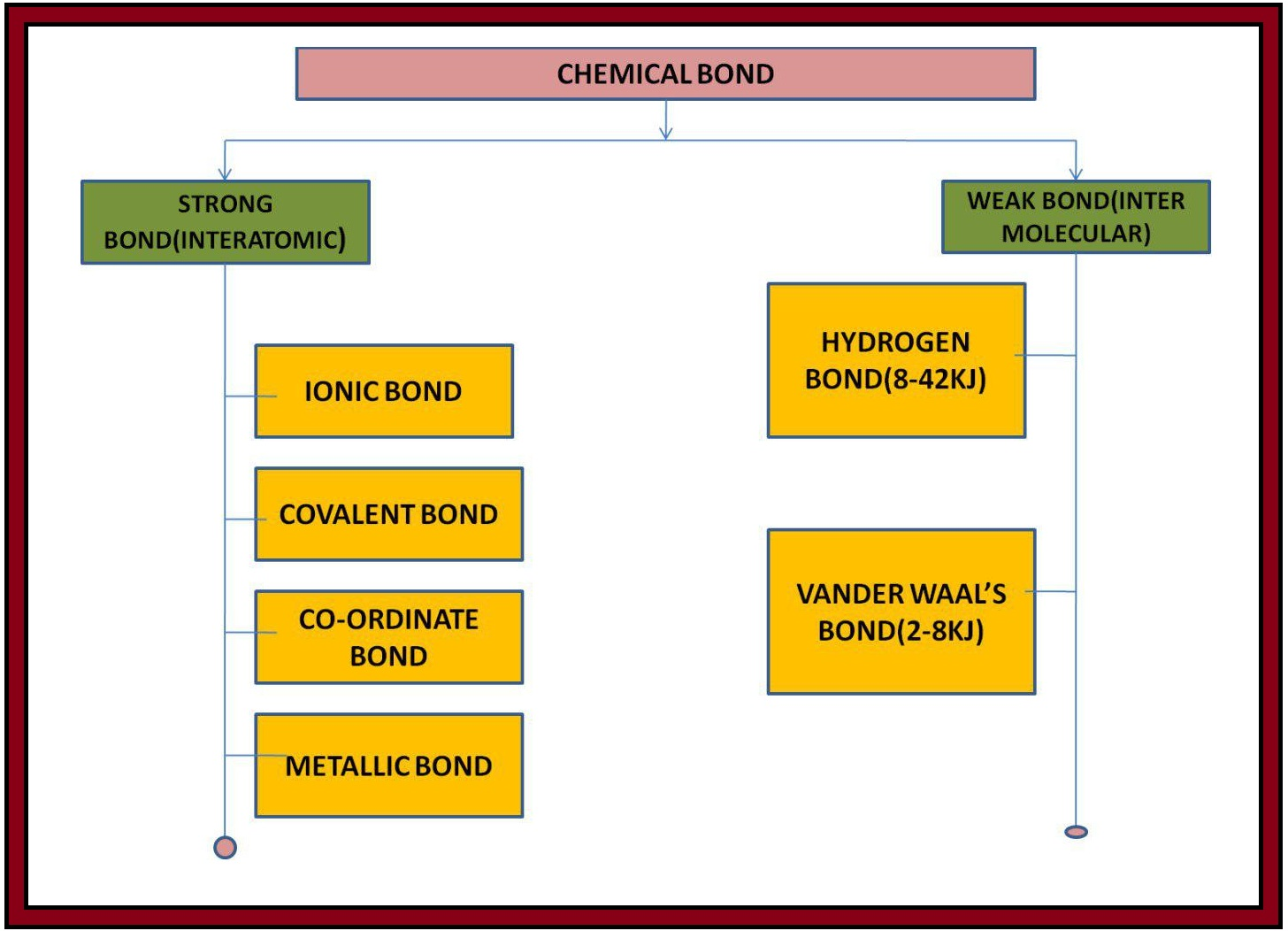
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
`color{green}["The attractive force which holds various constituents (atoms, ions, etc.)"]`
`color{green}["together in different chemical species is called a
chemical bond"]`.
`=>` Since the formation of chemical compounds takes place as a result of combination of atoms of various elements in different ways, it raises many questions like :
● Why do atoms combine?
● Why are only certain combinations possible?
● Why do some atoms combine while certain others do not?
● Why do molecules possess definite shapes?
The answers to these questions are given by Kössel-Lewis approach, Valence Shell Electron Pair Repulsion (VSEPR) Theory, Valence Bond (VB) Theory and Molecular Orbital (MO) Theory.
`=>` Under normal conditions no other element exists as an independent atom in nature, except noble gases.
`=>` However, a group of atoms is found to exist together as one species having characteristic properties. Such a group of atoms is called a `color{red}("molecule")`.
● Obviously there must be some force which holds these constituent atoms together in the molecules.
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
`color{green}["The attractive force which holds various constituents (atoms, ions, etc.)"]`
`color{green}["together in different chemical species is called a
chemical bond"]`.
`=>` Since the formation of chemical compounds takes place as a result of combination of atoms of various elements in different ways, it raises many questions like :
● Why do atoms combine?
● Why are only certain combinations possible?
● Why do some atoms combine while certain others do not?
● Why do molecules possess definite shapes?
The answers to these questions are given by Kössel-Lewis approach, Valence Shell Electron Pair Repulsion (VSEPR) Theory, Valence Bond (VB) Theory and Molecular Orbital (MO) Theory.